
- #Alternative balanced equation for the cracking of decane cracked#
- #Alternative balanced equation for the cracking of decane series#
- #Alternative balanced equation for the cracking of decane crack#
The geometry optimization and vibrational frequencies of reactants, transition states, and products are determined at the BH&HLYP/cc-pVDZ level, while energies are calculated at the CCSD(T)/cc-pVDZ level.
#Alternative balanced equation for the cracking of decane series#
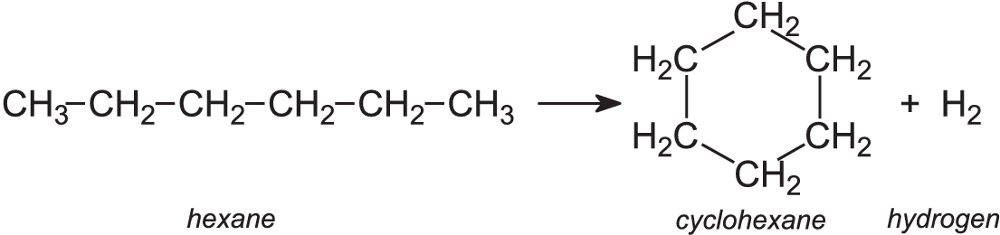
Three series of reaction schemes containing 38 elementary reactions are proposed. In the present work, the reaction mechanisms for thermal decomposition of cyclohexane in the gas phase have been investigated using quantum chemical calculations and transition-state theory. This constitutes an interesting and important area for further research. The effects of wall reactions of peroxy and peroxide species were not found to outweigh the impact of the main A-factors, but including wall losses led to significant higher order interactions between input parameters. The low temperature product channels for reactions of the cyclohexylperoxy radical are therefore an important area for future kinetic studies. At the low temperatures studied here, these are peroxy–peroxy radical reactions rather than the isomerisation routes that have been the subject of other investigations at higher temperatures. The HDMR study demonstrates a sensitivity of these features to the A-factors of key reactions of the cyclohexylperoxy radical (C6H11OO). The models also exhibit shorter induction times than those observed in the experiment. The kinetic mechanisms tested do not exhibit this characteristic when simulating the experimental conditions. The particular feature of interest is the characteristic of quadratic autocatalysis, which is observed experimentally and leads to the maximum rate of reaction occurring at 50% consumption of the deficient reactant (oxygen), with the fuel consumption exerting only a weak dependence. The analysis is used to investigate the important features of the oxidation process, as well as possible factors underlying qualitative discrepancies between simulations and experiments. This paper presents a global sensitivity analysis of simulations of low-temperature isothermal cyclohexane oxidation under fuel-rich conditions using the method of high-dimensional model representation (HDMR). In general, the calculation results provided the good evidence for the experimental phenomena that n-decane has larger cracking ratio than cyclohexane below 923 K. Therefore, n-decane was more prone to cracking than cyclohexane. The reaction rate constant for C-C bond cleavage of n-decane was larger than that for C-C bond and C-H bond cleavage of cyclohexane at the same temperature. At 1023 K, coke precursors, for example, aromatic compounds and cyclones, were generated in large amounts, which lead to the more serious coking of cyclohexane than that of n-decane. Further, the components of cracking products become more complex with the temperature increasing.
#Alternative balanced equation for the cracking of decane cracked#
Below 973 K, cyclohexane mainly cracked to form methyl-cyclopentane. The similarly high cracking ratio can be achieved at 1023 K, and major pyrolysis products of n-decane were C1-C4 hydrocarbons.

#Alternative balanced equation for the cracking of decane crack#
When the temperature is below 923 K, n-decane is easier to crack than cyclohexane, and major C1-C4 hydrocarbons and C5-C9 straight-chain olefins were produced. The results showed that under the same conditions, the starting temperature for the gase production from n-decane was lower than that from cyclohexane. To get the reaction rate constant for the initial key steps, theoretical calculation was performed by density functional theory method at BH&HLYP/cc-pVDZ level. To comparably analyze the cracking reactivity of these two model compounds, the cracking conversion, the gas yield, distribution of liquid and gaseous products were observed at the pressure of 4.5 MPa and in the temperature range of 823-1023 K. Pyrolysis of n-decane and cyclohexane under supercritical pressure was investigated by utilizing self-designed continuous flow reactor.


 0 kommentar(er)
0 kommentar(er)
